Aortic dissection, the consequence of a spontaneous aortic intimal tear, is usually due to a preexisting connective tissue disorder, hypertension, or vasculitis. Blood under arterial pressure enters and enlarges the potential space between the aortic intima and media, extending proximally and distally from the tear to potentially involve the great vessels an other aortic branches. Carotid, subclavian, renal, and mesenteric artery occlusion as well as aortic rupture are frequent complications. Dissection may be preceded by aneurysmal dilation, and persons with an aortic diameter greater than 3 cm are at increased risk.
Thoracic aortic dissections are classified by their point of origin, and this determines treatment and prognosis. Stanford type A dissections begin in the ascending aorta and may or may not extend into the descending thoracic aorta. These are managed surgically or by endovascular repair due to their high rate of fatal complications, which include acute aortic regurgitation, rupture into the mediastinum or pleural space, and cardiac tamponade from rupture into the pericardium. Stanford type B dissections arise beyond the left subclavian artery origin, do not require surgery, and are managed by aggressive blood pressure control.
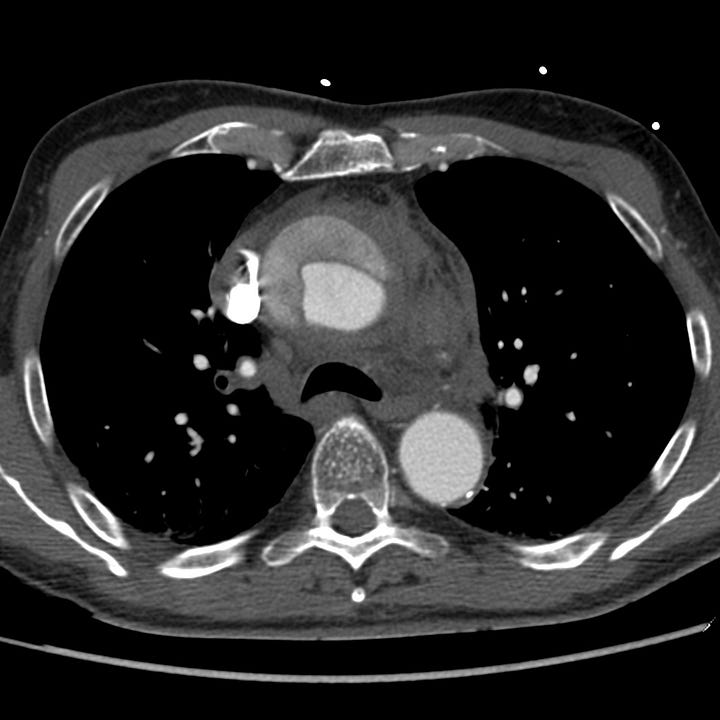
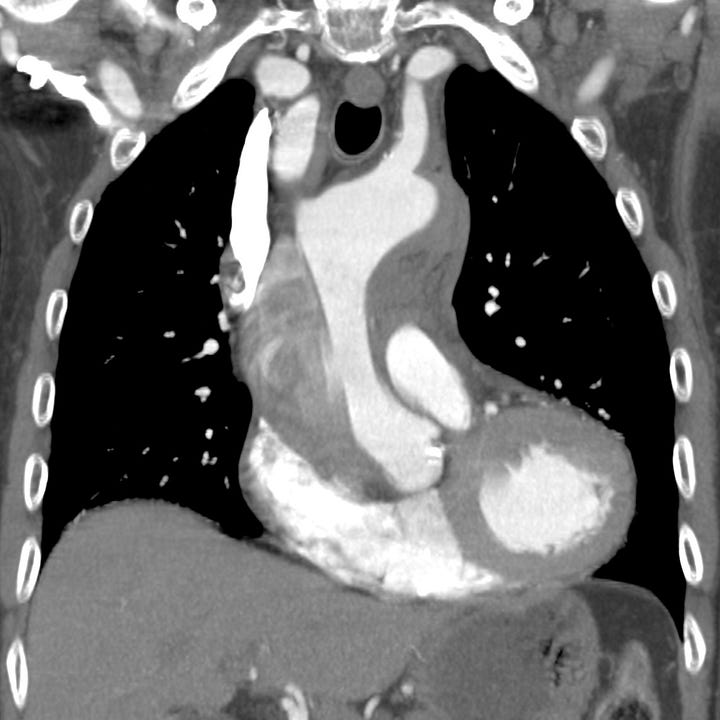
Type A aortic dissection. Unopacified blood and thrombus fills the larger false lumen of a proximal aortic dissection with associated mediastinal hematoma.
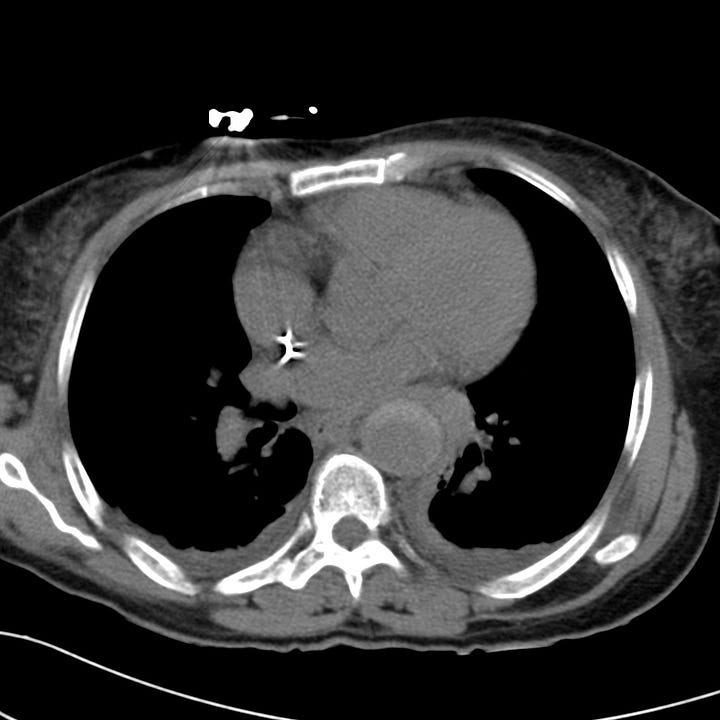
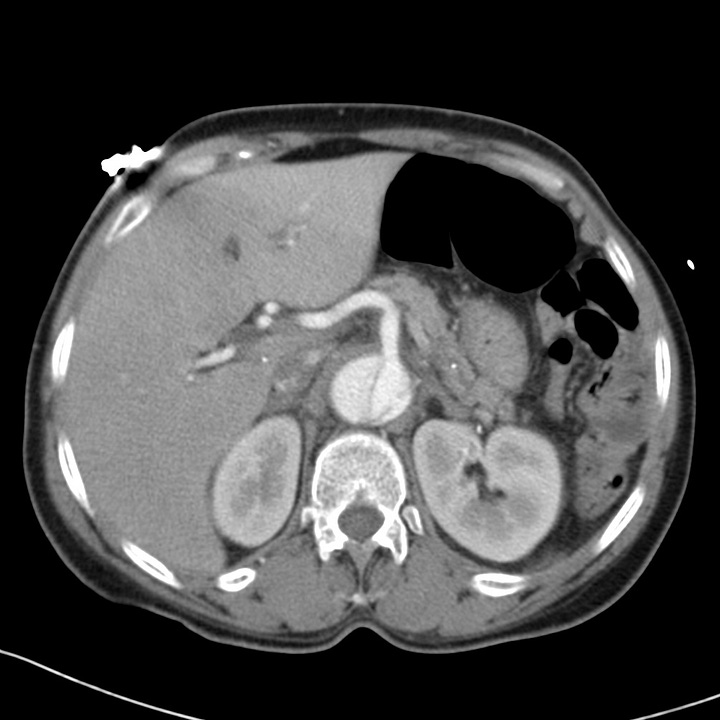
Type B aortic dissection. Acute hyperdense intramural hemorrhage surrounds the descending aorta on nonenhanced chest CT. CT angiogram at the level of the upper abdomen shows that the celiac trunk arises from the smaller true lumen.
Patients typically present with sudden, severe chest pain that radiates to the back. Occlusion of branch vessels may result in symptoms of renal, mesenteric or cerebral ischemia.
Urgent CT evaluation is indicated and should include a nonenhanced scan to identify hyperdense intramural or extra-aortic hematoma followed by arterial phase contrast examination. This permits identification of the true and false (intramural) lumens of the dissected aorta, as well as any branch occlusion, intraluminal thrombus, or focal dilatation.
Abdominal radiographs are generally not indicated and are often normal, but they may show aortic calcifications or aneurysm and, if compared with a prior examination, interval aortic enlargement or contour change.
Nontraumatic aortic rupture occurs in the setting of aortic dissection, aneurysm, coarctation, atherosclerosis, inflammation, mycotic disease, or erosion by an adjacent malignant tumor. Most such patients die from catastrophic blood loss, but some may survive to present to the emergency department with chest pain, hypotension and dyspnea.
As in traumatic aortic rupture, portable chest radiographs show a widened abnormal mediastinal contour. Indistinct aortic knob, widened paratracheal stripe, and depression of the left main bronchus are sometimes seen. CT is diagnostic and identifies aortic intramural hematoma, mediastinal pleural hematoma, hemopericardium, and other aortic abnormalities including dissection or pseudoaneurysm. Treatment requires urgent surgical intervention via open or endovascular repair.
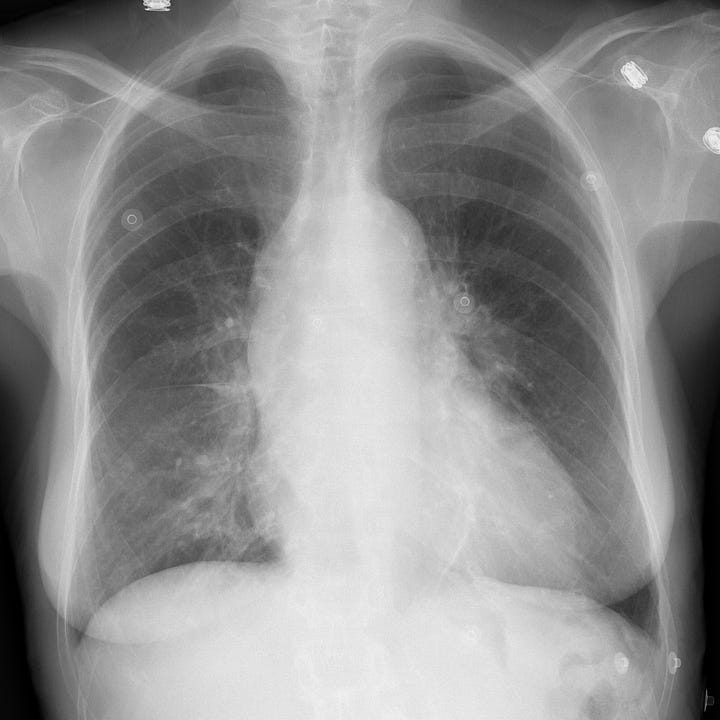
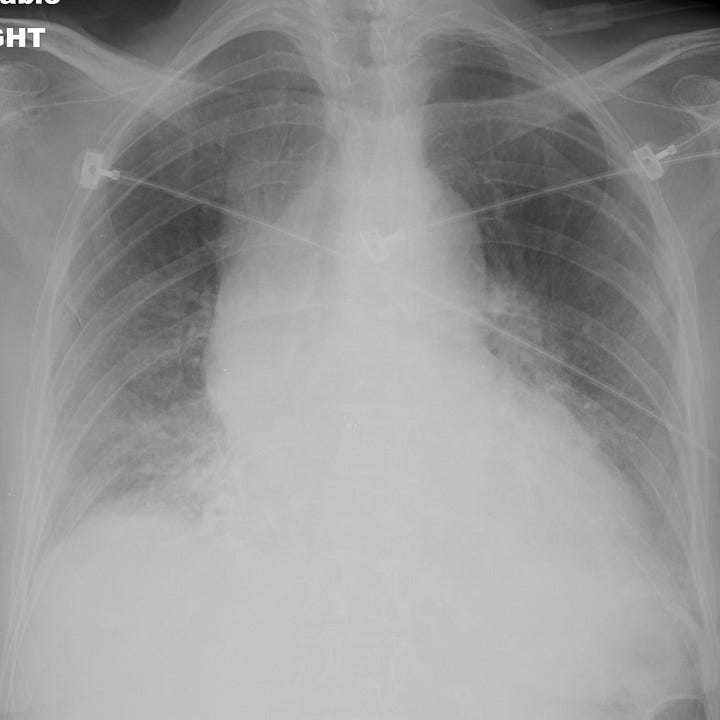
Nontraumatic aortic rupture due to ascending aortic (type A) dissection. Comparison radiograph from a prior admission shows baseline mild cardiomegaly. Portable examination obtained in the emergency department for evaluation of acute chest pain shows interval enlargement of the cardiac contour with marked ascending aortic widening.